Tuesday’s announcement from the FDA also included an update for people who are immunocompromised. They are now eligible for an additional booster, too. They initially received a series of three vaccinations and have already been eligible for one booster. Now, they, too, may get a second booster, meaning they can receive a total of five shots.
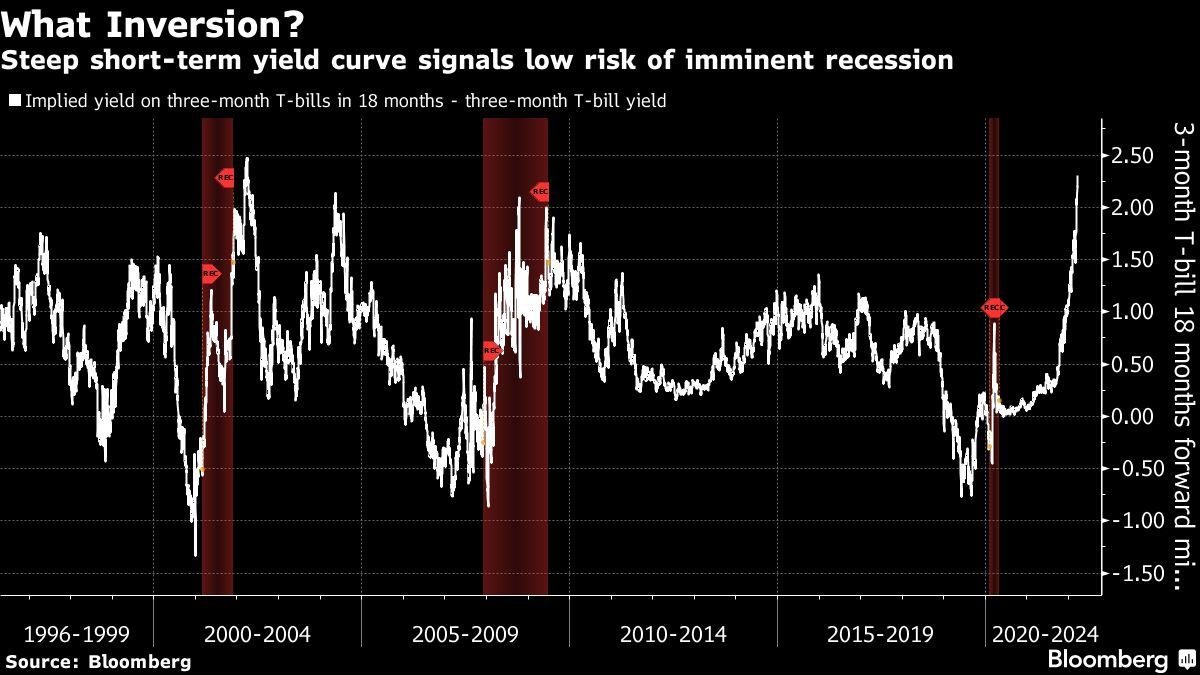
There are about 118 million people age 50 and older in the United States. But because not all of the people in that age range have received the initial series of shots or boosters, only a fraction are immediately eligible for a fourth shot.
Booster uptake in the United States has been slower than desired, particularly among older Americans who are at higher risk of severe illness. About 15 million people age 65 and older — a third of people in the age group — are fully vaccinated but have not gotten a first booster. Only about 40 percent of the people between the ages of 50 and 64 have received a first booster.
“I would urge people to get their first booster because one thing that did become apparent … is the third dose provides a differentiating level of immunity that does seem to provide people some additional benefit, in terms of preventing the severe outcomes of hospitalization and death — and that seems to last and be more durable,” Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said at a news briefing.
The second booster for adults 50 and older and for people 12 and older who are immunocompromised is expected to become available immediately after the Centers for Disease Control and Prevention reaches a decision on who should get it.
The CDC is expected to say that people in the age group may get a fourth shot, instead of an explicit recommendation that they do so, a reflection of the ongoing debate about the benefits of additional doses and uncertainties about the future of the pandemic.
The messenger RNA boosters will be available to people regardless of which brand of coronavirus vaccine they initially received, including Johnson & Johnson recipients. The Pfizer-BioNTech booster is authorized for immunocompromised people age 12 and older. The Moderna vaccine has not yet been authorized in teenagers, and its booster is authorized for immunocompromised people 18 and older.
Experts, who have been inundated with questions from family members and emails from the public seeking advice, said they hoped the agency would give clear guidance to help people and health-care providers navigate the situation.
“I’ve been getting multiple questions from lay friends over the past few days: ‘What does this mean, and what should I do?’ ” said John P. Moore, a professor of microbiology and immunology at Weill Cornell Medicine in New York. “I find it increasingly difficult to tell friends what they should do. It’s becoming really problematic.”
The primary benefit of a fourth shot is thought to be protection against severe illness, and that risk can vary dramatically among people 50 and older. Not all experts are convinced the benefits are clear, and some have debated about whether the age cutoff should be 60 or older. A matrix of factors — including underlying health conditions, age, and time since last booster dose or infection — could play a role in what a person should consider in risk vs. benefit.
A 70-year-old with diabetes and high blood pressure, for example, who received a booster dose in October probably would be at dramatically different risk from covid-19 than a 50-year-old with no underlying health conditions. Tens of millions of people were infected with the omicron variant during the winter surge, and those people’s immune systems have been effectively boosted — so they may not need another shot now.
Some experts have raised concerns about the decision-making process. The second booster issue is not scheduled to be presented to outside advisory committees of the FDA and CDC.
“I think it’s wonderful that the FDA is making [a second booster] available,” said William Schaffner, an infectious-diseases expert at Vanderbilt University and a liaison member of the CDC’s Advisory Committee on Immunization Practices. But Schaffner said such decisions are typically reviewed by the CDC’s advisers, “and I think that mechanism should have been used for this also.”
Even if outside experts reached a similar conclusion, having a discussion and seeing data presented at a public meeting offers transparency and a “different tone,” Schaffner said.
But the FDA news is certain to be welcome to a segment of the population “who will be at their doctor’s office or pharmacies tomorrow, if not this afternoon, getting their fourth dose,” Schaffner said. “But they will be the minority of the population because after all, before you get your fourth, you have to get your third,” Schaffner said.
Evidence in support of a fourth dose is limited and mixed, based largely on data from Israel — where people 60 and older have been able to receive a second booster shot. Israeli studies have supported the idea that an additional shot could be lifesaving for people older than 60 but have also suggested the shots will have marginal benefits for young, healthy people and offer only short-term and modest protection against getting infected.
One preprint study from Israel found a 78 percent reduction in death among people older than 60 who received a fourth shot, compared with those who received only three shots. Another preprint found a lower rate of cases of severe illness among people older than 60 who received four shots, but was limited, covering only a two-week period in late January.
Data included in a letter to the editor of the New England Journal of Medicine provided a more mixed picture. Among health-care workers in Israel, a fourth shot increased virus-blocking antibodies. But that boost provided little protection against infection, and people who became infected experienced few symptoms regardless of whether they had received three or four shots. Vaccinated people had relatively high amounts of virus in their nose, suggesting they could pass the infection to others.
The Israeli data underscored that boosters’ effects are transient and that a fourth-dose strategy is a short-term approach. An Israeli preprint study published last week before peer review found that a fourth dose was 73 percent protective against severe illness compared to three doses over the course of 10 weeks of follow-up. But the protection against infection was modest and short-lived, peaking at 64 percent three weeks after the vaccination and falling to about 29 percent.
Marks acknowledged the data supporting a fifth dose for immunocompromised people was more limited, but said that it was seen as a way to protect vulnerable people. Vaccines tends to generate weaker protection against covid-19 in this group of people and tends to erode more quickly.
Timing the shots’ peak protection to the time of greatest risk from the virus is tricky. No one knows when future variants will emerge, and scientists are uncertain even about known threats. Infections from the BA.2 version of the omicron variant are ticking upward in the United States, but some health officials have said they don’t expect BA.2 to cause a surge. Some experts predict a surge next winter.
In terms of giving a second booster, Moore said, “Should you do it now — and in the fall? Or in the fall—and not now? This is where it gets head-spinning. What is the long-term intent, and what is the long-term policy?”
Separate from Tuesday’s action, the FDA plans next week to convene external advisers who will debate the long-term booster dose strategy for the general population. One possible scenario, Marks said, is a fall booster campaign that coincides with annual influenza shots.
Vaccine makers are working on shots they believe will provide more lasting protection. In recent days, leaders from Pfizer, and German partner BioNTech, and Moderna have indicated they believe new versions of their vaccines, including formulations that incorporate two versions of the coronavirus, will create longer-lasting immunity.
“I think about my family — my loved ones, and what I would say. … I would probably tell them to just kind of hang back until there’s clear evidence of a rise in their area,” said Natalie Dean, a biostatistics expert at Emory University’s Rollins School of Public Health in Atlanta. “There’s something to the timing of it — and where numbers remain low and they’ve been boosted not that long ago, a few months ago, I wouldn’t go out and tell them they need that right now.”
Dan Keating and Lena H. Sun contributed to this report.














































































































































































































































































































































































































































































![“Ghosts” recap: season 2, episode 10 – [Spoiler] To kiss](https://nokiamelodileri.com/wp-content/uploads/2022/12/Ghosts-recap-season-2-episode-10-Spoiler-To-kiss.jpg)





































































































































































































































































































![The Walking Dead Recap: Season 11, Episode 23 — [Spoiler] Die?!?](https://nokiamelodileri.com/wp-content/uploads/2022/11/The-Walking-Dead-Recap-Season-11-Episode-23-—-Spoiler.jpg)









































































































































































































































































































![‘She-Hulk’ Recap: Finale Criticizes MCU Tropes, Introduces [Spoiler]](https://nokiamelodileri.com/wp-content/uploads/2022/10/She-Hulk-Recap-Finale-Criticizes-MCU-Tropes-Introduces-Spoiler.jpeg)












































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































![‘I mean, I kinda agree [with] them’](https://nokiamelodileri.com/wp-content/uploads/2022/03/I-mean-I-kinda-agree-with-them.jpeg)








































![TikToker Admits He Lied About Jumping That Tesla to Go Viral [UPDATED]](https://nokiamelodileri.com/wp-content/uploads/2022/03/TikToker-Admits-He-Lied-About-Jumping-That-Tesla-to-Go.jpg)


































































































































































































































































































































































































































































































































































































































![‘Good Trouble’: [Spoiler] Leaving in Season 4](https://nokiamelodileri.com/wp-content/uploads/2022/03/Good-Trouble-Spoiler-Leaving-in-Season-4.jpg)














































































































![‘The Bachelor’ Recap: Season Finale—Clayton Picks [Spoiler]](https://nokiamelodileri.com/wp-content/uploads/2022/03/The-Bachelor-Recap-Season-Finale—Clayton-Picks-Spoiler.png)











































































































































































































































































































































































![‘Upload’ Recap: Season 2 Premiere, Episode 1 — Ingrid Is [Spoiler]](https://nokiamelodileri.com/wp-content/uploads/2022/03/Upload-Recap-Season-2-Premiere-Episode-1-—-Ingrid-Is.jpg)





















































































































































































![[VIDEO] ‘The Masked Singer’ Premiere Recap: Season 7, Episode 1](https://nokiamelodileri.com/wp-content/uploads/2022/03/VIDEO-The-Masked-Singer-Premiere-Recap-Season-7-Episode-1.jpg)



























![‘The Bachelor’ Recap: Fantasy Suites, Clayton and [Spoiler] break-up](https://nokiamelodileri.com/wp-content/uploads/2022/03/The-Bachelor-Recap-Fantasy-Suites-Clayton-and-Spoiler-break-up.png)




















































































0